There is a lot of confusion and misinformation about the importance of the acidity / alkalinity of the food we eat. There are a lot of people that spend a lot of effort (as well as money) on “alkalizing the body”. This is taking measures to force a change in the body’s pH to make it more alkaline (or base). First let me say that this topic is covered quite well in The Gut Health Protocol. And second, let me just re-emphasis that trying to alkalize the body is a very bad idea.
“The food chyme leaving the stomach is very acidic at pH 4-5. Intestinal pH gradually increases in the small intestine until it is at about pH 6 at the bottom of the duodenum. The pH drops to 5.7 in the caecum (the beginning of the large intestine), but again gradually increases, reaching a pH of 6.7 in the rectum. These levels are quite a bit more acidic than blood which has a pH of about 7.4. This lower, more acidic, pH is needed to hold down candida growth and promote probiotic bacteria growth.” — “The Gut Health Protocol”
I sometimes wonder if this entire argument is simply one of semantics. People (including some doctors I’m afraid) are getting an “Alkaline pH” confused with our need for “alkalizing minerals”. Providing the body with plenty of “Alkalizing” nutrients is wonderful and very important. Making the body more alkaline is very dangerous. Do alkaline foods contain a lot of alkalizing minerals? Sometimes, but so can acidic foods; in fact grass-fed beef contains more alkalizing minerals per ounce than fruit and vegetables.
This topic has become almost religious in nature. So much so that fights break out and people get banned from online forums and Facebook groups. So biased that many of the charts you find on the internet simply make up their facts. These graphs vastly misrepresent the pH of foods, making some foods far more acidic than they really are (such as beef and pork) and making other foods far more alkaline than they really are (such as spinach and broccoli). I’m looking at a chart as I type this that has beef and pork at a pH of 3.0-4.0, yet the real pH of these meats is actually a pH of 5.8 (1, PubMed #11263828, PubMed #PMC1805706). They show the pH of spinach and broccoli to between 10.0 and 11.0! This would be very alkaline, however, this is not the case, the real pH of spinach is 5.5 (fda.gov). The pH of spinach is actually more acidic than beef. Yet both numbers were fudged on many charts that I looked at to make it look just the opposite. But this wasn’t just limited to a couple of foods, but to nearly every food on several different charts (actually every “alkalizing” chart I looked at, and Iooked at over a dozen, fudged their numbers). Here are a few more examples:
- Blueberries were listed at ph 9-10, their actual pH is 3.2! Which is very acidic and is a 30x difference! (every 1.0 increase in pH is 10x more alkaline).
- Beats were listed at pH 9-10, their actual pH is 5.5
- Flour was listed at a pH of 4.0, it is actually 6.2
Some of these charts were put out by companies selling alkalizing water machines, addons to reverse osmosis machines, etc. There were many other charts from probably well meaning bloggers who were most likely getting their information from these same companies. Some of the authors of these charts obviously knew this information was phoney because in the fine print they said that “This chart reflects the particular food’s on the pH balance in the human body which may or may not reflect the direct pH value of the food itself”. There are several attempts out there to try to show certain foods in a good light, and other foods in a bad light, they do this by making up this functions that have no basis in science, such as certain foods produce “alkalizing ash” in the body. This hypothesis is being pushed to sell these products, and to try to dissuade people from eating animal protein and consuming dairy. It has nothing to do with science. What little science that has been used to prove this hypothesis has been extremely poor and is easily argued with much stronger evidence and higher quality studies.
Fruits are actually very acidic, but they also contain some buffering minerals. The acidity of the fruit you eat will be shuffled off by the kidneys into your pee, or to your lungs to be exhaled as carbon dioxide. The minerals will stay behind to help buffer (neutralize) the blood’s pH as necessary. But so will the acidity of beef, dairy, flour, etc. If you are really looking for a good boost of buffering minerals you would go with a good serving of beef liver or kidney, it is far higher in buffering (alkalizing) minerals (per ounce) than any fruit or vegetable, it also less acidic than many fruits and some vegetables. So if you truly believe in the alkalizing hypothesis then eating beef, especially organ meat, is the way to go.
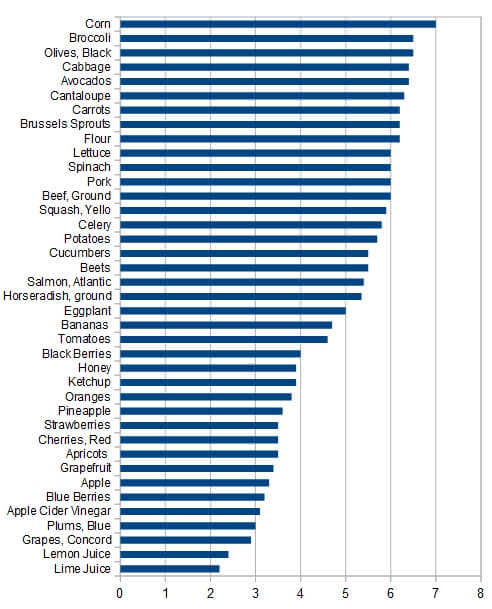
pH of various foods, © John Herron
“The concept that the modern diet produces excess acid, which causes several diseases of modern societies, and that “alkaline diets” prevent and cure these diseases are marketed to the general public across the globe. The public is being encouraged to measure their urine and/or salivary pH to assess their health status and their risk of disease…A causal association between dietary acid load and osteoporotic bone disease is not supported by evidence and there is no evidence that an alkaline diet is protective of bone health.” — PubMed #PMC3114717
Before you start thinking that all of these studies are in bed with the pharmaceutical companies this study from The UK Food Standards Agency supports the recommendation for more fruit and vegetables, but also concludes that it has nothing to do with the pH of the food, alkalizing the body, or the acidity of the food: “promoting a diet rich in fruit and vegetable intakes might be beneficial to bone health and would be very unlikely to produce adverse consequences on bone health. The mechanism(s) for any effect of fruit and vegetables remains unknown, but the results from these projects did not support the postulated acid-base balance hypothesis.” — PubMed #18086331
There is also much talk on popular health websites / blogs about how bad meat, especially beef, is for our bone health; often citing the “acid forming” characteristics of beef. This study debunks that as well – “dietary proteins are as essential as calcium and vitamin D for bone health and osteoporosis prevention. Furthermore, there is no consistent evidence for superiority of vegetal over animal proteins on calcium metabolism, bone loss prevention and risk reduction of fragility fractures.” — PubMed #16373952
“based on quality randomized trials and prospective observational studies, did not find support for the acid-ash hypothesis which states that “acid” from the modern diet causes osteoporosis or that an alkaline diet or “alkaline” supplements or salts prevent osteoporosis.” — PubMed #PMC3114717
Nearly every study that tried to support an “acid-ash hypothesis” was either very small, nonrandomized, used highly unrealistic acid levels (e.g. included twice the upper tolerable limit for salt), no equalization of minerals between the study group and the controls, or more than one of these factors. No well run studies have been done on the “acid-ash hypothesis” that concluded with supporting evidence of the hypothesis.
Many people go through extremes to try to raise their pH (make it more alkaline), including purchasing alkalizing machines, testing their urine, giving up meat, dairy and other proteins, purchasing expensive nutrients, etc. Some people even end up drinking solutions of baking soda to try to further this end. The body needs high levels of protein intake, when it doesn’t get it from the diet it will cannibalize muscle tissue to obtain the protein that it needs. Either consuming protein in the diet, or cannibalizing muscle tissue will cause urine to be more acidic. This often causes people to be frustrated and try even harder to alkalize their urine. Eventually they succeed but at a great cost to the body.
The intestine needs to be acidic, there is no doubt about that in science. Doing anything to make the gut alkaline will cause disease. The key is simply to eat nutritionally, lots of mineral rich foods, then you never have to worry about the pH of your food (except too much acidic citrus can damage your teeth wink emoticon There is always an exception).
Restricting foods simply because they are “acidic” is a bad idea, they can contain a lot of nutrition and the acidity of the food makes very little or no difference to overall health. Worrying about the pH of your urine is a bad idea. If the last three meals you ate were full of buffering minerals, mineral rich vegetables / fruit, acidic grass-fed beef, acidic citrus fruit, then your urine’s pH may very well be acidic. But that is meaningless for determining health. What is important is that you consumed a lot of “alkalizing minerals”. These minerals are used by your body to buffer acids, if you have enough of the various buffering agents in your body acidic food is not bad for you in the least, assuming it is healthy mineral/vitamin rich food. Trying to artificially alkalize your body to offset this rise in urinary pH is very unhealthy, unnatural, and likely to lead to increased pathogen growth (see below).
“It is well established that a pH around neutrality favours hyphal development of C. albicans (candida albicans) in vitro, while a low pH (pH < 6.5) blocks hyphal formation… One of the major virulence factors is its ability to switch between yeast and filamentous form.… A pH of 5.4 also induces low filamentation, pH 6.4 gives moderately lower than pH 7.4. A pH of 7.4 was best suited for germ tube induction.” — ojmm.2013.33028
Dr. Chris Kresser — “The Acid-Alkaline Myth”, Part 1. http://chriskresser.com/the-ph-myth-part-1/ The whole theory that it creates ” ‘ash’ after they are metabolize” is nothing more than myth.
Bicarbonate is very important in the body, but as a buffering agent. It is made by the body and stored in order to adjust the pH on-demand. For this reason it may very well have some benefit in preventing cancer. Just like calcium, magnesium, and potassium as all are buffering agents (they also have other roles). However, the main job of bicarbonate in the body is to regulate the pH of the blood and to raise the pH of food leaving the stomach and entering the small intestine. You do NOT need to consume sodium bicarbonate for this to occur. The body is perfectly capable of making all the sodium bicarbonate it needs from CO2 in the air we breathe. We do NOT have a bicarbonate deficiency and we do not need to supplement it (heck with global warming we have more CO2 in the air we breathe than ever).
“Despite significant effects on the formation of metastases and tumor pHe, chronic bicarbonate therapy had no effect on blood chemistries, indicating that systemic pH was fully compensated in these animals (Supplementary Table S1). Thus, as expected due to the chronic nature of the treatment, NaHCO3 (Sodium bicarbonate) did not lead to systemic metabolic alkalosis. Rather, we hypothesize that inhibition of tumor metastasis was due to increased bicarbonate “buffering” of interstitial fluid of either the primary or the metastatic tumors. ” — http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2834485/
Again, it isn’t the pH that matters, it is the buffering potential. Thus we shouldn’t be worried about the pH, we should be worried about obtaining plenty of good minerals in the food we eat. This is the point I’ve been trying to make, we shouldn’t worry about the pH of the food we eat, we should worry about the minerals it contains. The good news in this argument is that regardless of which side of the argument you take you should be eating more vegetables and low sugar fruit. But if you really want good health you will cook those vegetables (for far better bioavailability of those minerals) and consume nutrient dense animal proteins.
This video is also pretty good at explaining this.
See YouTube for information about this video
All images posted by John Herron are either "Copyrighted John Herron", or are copyrighted by someone else and are used under license. So please don’t use them elsewhere, you’ll get in trouble.

 Phage Complete comes with a full 30 day money back guarantee, for U.S. purchases this includes the original shipping charges to you!
Phage Complete comes with a full 30 day money back guarantee, for U.S. purchases this includes the original shipping charges to you!
Hi John-thank you for this post and your excellent book. Do you ever do consulting with people around digestive issues? Could you let me know via the email address below? Thanks very much. I’d be happy to pay a fee. Erik
Thank you for your post. I am dealing with sibo and was wondering if apple cider vinegar daily would kill bad bacteria.
No, Apple Cider vinegar will get buffered as it enters the small intestine (just like stomach acid in the food chyme) and will have little to no affect on gut bacteria.
Animal proteins also have a higher concentration of sulphur containing amino acids that get metabolized to acid-generating metabolites. As a result, a slightly lower physiological pH must be corrected and buffers like calcium are used to attenuate these adverse acid effects–to the disadvantage of the host.
All proteins are made up of acids, amino acids, and have to be buffered. This is a very normal biological function for the body.
Sorry a few typos, should read
Hi John, this has been a revelation, I have spent years wondering why everytime I take bicarb for a urianry tract infection my gut becomes unstable and the only thing that helped was a recommendation from a naturapath who suggested (after doctor after doctor put me on antacids making me worse) that I need more HCL not less and whithin hours started recovering. Seems so obvious now, question though how does one alkalise with the minerals you mention without affecting the acid levels of the gut?
If absorption and assimilation are good a lot of the minerals will be absorbed and not enter the gut. But this is why would should also be consuming acidic foods, such as citrus and meats. This will help balance the pH. The body also worked to keep the pH in balance.
Wow very few know this. I have been trying for 2 years to explain this to people. messing with the ph of the body can cause a variety of issues and cause a variety of kidney stones to form.
You trust the FDA and their data? We’ll end the discussion there. Just remember that the pH of a ripe vs. unripe blueberry is *very* different.
The pH of food is not something the FDA can lie about. Every university and country can run the same tests. Conspiracies for something so easy to verify are not possible.
But in reality it doesn’t really matter. Much of our food is acidic. For example, all proteins (animal or plant based) are made up of amino acids, acids are, of course, very acidic. What is important is consuming enough minerals so that our body can buffer these acids without robbing minerals from our bones. Normally even this isn’t a problem as the body has other ways of ridding itself of the excess acid (e.g. urine, converting it to CO2 and expelling it through the lungs, etc., etc).
Health wise, the pH of our food is really one of the last things most people need to worry about. This is considering a very long list of worries.
I agreed because i actually get respond from my body as i ate these food listed and get result like beef etc depend on how they are cooked
was on raw veggie and i had more issue
i like watermelons
https://eurekalert.org/pub_releases/2018-04/mcog-dbs042418.php
John, that is a good link on fighting autoimmune disease, thank you. I’ve been sodium bicarbonate lately in regards to cancer, this fits in with the anti-inflammatory properties.