
Phage Complete – Benefits
Phage Complete is the most advanced Probiotic / Prebiotic / Phage formula on the market today. A proprietary formula based on the latest scientific research, every ingredient has been chosen to heal and benefit the gut. Some of the strains have been scientifically shown to benefit the gut after taking antibiotics. Though this is a fairly long document I think you’ll find it very informative. Phage Complete is based on thousands of research studies to bring you a supplement designed from the ground up to help heal and benefit the gut and the immune system, the keys to good health. You can click here to order, or here to read an article on phages in general.
 Click here for many more testimonials!
Click here for many more testimonials!
Phage Complete:
- Contains a completely safe phage complex that is scientifically shown to selectively kill certain strains of “bad” bacteria, does not harm our beneficial bacteria, and supports
 a healthier microbiome. The cells of dead bacteria serve as a prebiotic food for our beneficial gut bacteria! PreforPro® supports the growth of beneficial bacteria in the gut through a novel prebiotic that’s not fiber or starch-based, and requires a significantly smaller dosage than typical prebiotics.
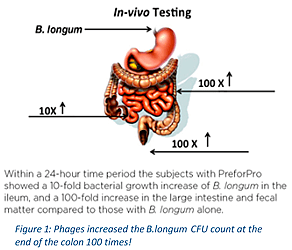
a healthier microbiome. The cells of dead bacteria serve as a prebiotic food for our beneficial gut bacteria! PreforPro® supports the growth of beneficial bacteria in the gut through a novel prebiotic that’s not fiber or starch-based, and requires a significantly smaller dosage than typical prebiotics. - Scientifically shown to vastly amplify the number of beneficial bacteria in the colon and help rebalance the bacterial microbiome of the small intestine. See figure 1.
- Shown to help the body balance the microbiome, which studies show can improve several conditions, such as: leaky gut, bacterial overgrowths, acquired fructose malabsorption, intestinal inflammation, bloating, and thought to benefit any condition where the microbiome is involved.
 Shown to significantly improve the immune system.
Shown to significantly improve the immune system.
“The gastrointestinal system plays a central role in immune system homeostasis… The crucial position of the gastrointestinal system is testified by the huge amount of immune cells that reside within it. Indeed, gut-associated lymphoid tissue (GALT) is the prominent part of mucosal-associated lymphoid tissue (MALT) and represents almost 70% of the entire immune system; moreover, about 80% of plasma cells [mainly immunoglobulin A (IgA)-bearing cells] reside in GALT.” – PubMed ID#PMC2515351
- Contains Bacillus subtilis a spore forming bacteria normally found in a healthy gut. This strain can persist in the GI tract, increase its numbers and then re-sporulate. The phage complex enhances this process.
- Delayed Release capsule ensures probiotics travel safely through the harsh conditions of the stomach to where they belong, in the intestines.
- Very gentle on you, yet starts working in hours! No bloating fibers or prebiotics.
- Compare Phage Complete to other products; Phage Complete contains NO Maltodextrin, NO GMO Ingredients, NO Glycerin, NO Magnesium stearate, NO Gluten/Eggs/Soy/Corn/Nuts/Dairy/Lactose/Sugar* and is Vegan diet safe. Few other products can say this!
- One bottle of Phage Complete is a two-month supply for most people and costs significantly less than similar probiotics (two capsules per day can be taken during the kill and healing phase). We invite you to compare Phage Complete to the probiotic you’ve been taking.
- Non-histamine producing probiotic strains, this formula does not cause histamine related inflammation / reactions. This greatly benefits healing as many people with intestinal issues are sensitive to histamine. Inflammation leads to leaky gut and both inflammation and leaky gut can cause many symptoms and slow healing. In fact some of the strains in Phage Complete actually help degrade histamine or help in the healing of histamine intolerance conditions.

Independently certified by one of the world’s largest FDA approved certification companies. - No d-lactate (d-lactic acid) producing probiotic strains. Most probiotics contain strains that produce significant amounts of d-lactate and this can cause a range of symptoms for many people. See “Lactic Acidosis – Fatigued, Confused, Grumpy? This might be why.”
- The probiotic strains in this formula produce natural vitamins (including Vitamin K2 and folate) that are easier to absorb and utilize than synthetic vitamin pills. This can also benefit peoplewith certain genetic conditions (such as MTHFR) where certain biochemical pathways, that convert synthetic vitamins to their natural form, may be hindered.
- FODMAP Diet safe formula, contains no FOS, inulin or other bloating prebiotics. These particular fibers are the “F” in FODMAP. Some probiotic formulas also contain glycerin, which is a polyol, polyol is the “P” in FODMAP. Phage Complete contains no FODMAPs and does not cause gas, bloating or flatulence.
- No refrigeration required, 11 Billion CFUs at time of manufacture, formulated to maintain at least 7.5 billion CFUs after 18 months (from time of mfg) under normal shelf conditions. Because of the prebiotic phage complex included in this formula, a higher probiotic CFU count would not be recommended. See further details below.
- Designed to improve the benefits of other probiotics and fermented foods. The CFU increase of some probiotics has been measured at x100 or more compared to the same probiotic without phages. See Figure 1. Consuming other probiotics with a high CFU count (especially histamine and d-lactate producers) is never recommended.
- Microbial imbalances are linked to many different diseases and conditions. This includes, but is not limited to: diarrhea, constipation, “brain fog”, depression, anxiety, fibromyalgia, chronic fatigue, poor energy levels, IBS, SIBO, autoimmune conditions, and many diseases normally linked to the immune system. Balancing the microbiome can go a long way in helping your body get rid of these conditions or prevent them in the first place.
- Starts working in hours, not days or weeks. There is a 30-day money back guarantee, if you haven’t started to see some positive results in 30 days return it for a full refund to the address on the bottle. Please include a copy of your PayPal receipt, all refunds will be made through PayPal (either to your PayPal account or the credit card you used to purchase).
- Less than $1 / day
- Formulated by the author of The Gut Health Protocol and Cancer: Improving Your Odds (with assistance from some serious researcher scientists!)
- Manufactured in a USA Good Manufacturing Practices (GMP) facility, independently inspected by NSF.
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
Comparison Table
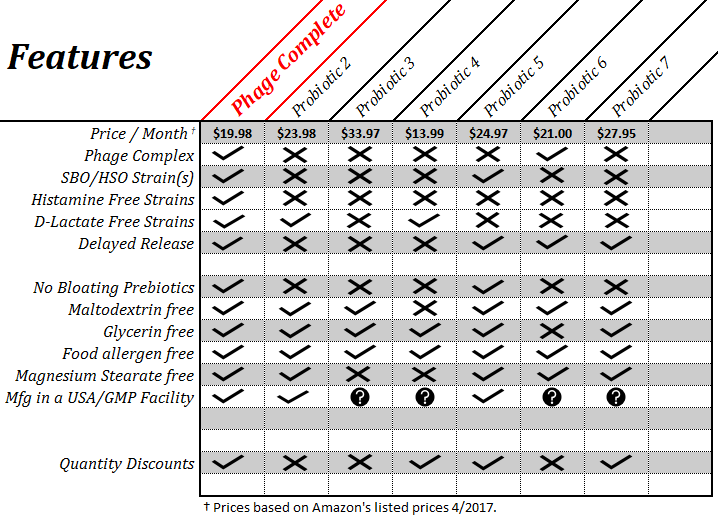
The table above compares Phage Complete to 6 other popular probiotics; big names and popular online doctor brands. As you can see Phage Complete is not only less expensive than all but one probiotic, but beats all of them in the comparison. People trying to heal their gut should take 2 capsules of Phage Complete per day for a limited time, this can be lowered to 1 capsule after gut symptoms improve. This may not be advised for some of the other formulas due to unwanted ingredients or poorly selected probiotic strains.
Are you ready to order? If so, Click Here
When comparing probiotics, you should not look at just the number of strains and CFUs. Phage Complete contains fewer strains and CFUs than some of the other probiotics, but it would have been very easy, and inexpensive, to add more Lactobacillus and Bifidobacterium strains to the formula. However, this would have meant adding strains that produce histamine, create harmful d-lactate acid, break down bile acid, or competed with the other good bacteria while serving little good. The strains in this formula were chosen to provide the greatest benefit, while not leading to harmful inflammation from subclinical histamine overload and other issues. A higher CFU is not always better and can often cause unwanted side effects.
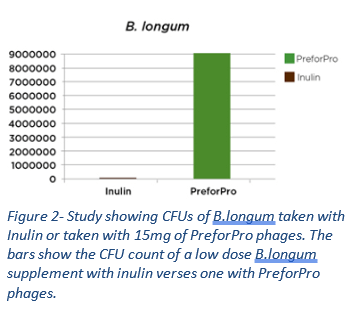
As shown in Figure 2, standard probiotic formulas usually do not benefit the lower regions of the colon, even if taken with inulin. The phage complex in Phage Complete (PreforPro™) not only feeds beneficial bacteria but also selectively kills their unwanted competition; the results are dramatic. It does this without the bloating and gas associated with inulin and many other prebiotic fibers.
Phage Complex
The phage complex included in this formula has been shown to amplify the benefits of many probiotic bacterial strains. Phages work like a very selective kill supplement as well as a highly effective prebiotic. Yet it does this without the side effects of either traditional kill supplements or fiber/starch based prebiotics.
The following bacteria showed significant growth when this phage complex was co-administered, compared to the control: L. rhamnosus, B. bifidum, B. longum, B. subtilis. These strains are also included in Phage Complete. These strains all have benefits to gut health, as described below.
Phages are absorbed by their matching bad bacteria, destabilizing (lysing/breaking up) the bacterial cell wall, resulting in release of nutrients into the gut environment which can then be utilized by probiotics and the good bacteria of the GI tract. FDA regulations do not allow the manufacturer (or us) to market supplements in a way that infers a disease treatment; if you have truly pathogenic bacteria you should always follow your doctor’s directions. The phages in PreforPro® have been granted Generally Recognized as Safe (GRAS) by the FDA and are commonly found in our environment (they are not GMO).
“the reduction of E. coli, decreased proportions of potentially pro-inflammatory bacteria, and increases in fermentative taxa that are capable of butyrate production suggest a shift toward a healthier gut environment. As gut dysbiosis continues to be associated with human disease and the medical community is confronted with anti-microbial “superbugs”, bacteriophages offer an additional resource to combat these issues… it is plausible that the phage treatment resulted in lower circulating LPS, which may drive reductions in Il-4 (a cytokine associated with inflammation)” — PubMed #PMC6471193 – Colorado State University “PHAGE” Study
As well as killing E.coli bacteria the PHAGE and PHAGE2 (recently released) studies found that the phage complex (found in Phage Complete) also dramatically decrease strains Citrobacter and Desulfovibrio, which are associated with gut inflammation and GI disorders. Both of these are hydrogen sulfide (H2S) producing bacteria. It also decreased Clostridium perfringens, a gram-positive, rod-shaped, anaerobic, spore-forming pathogenic bacterium of the genus Clostridium. The study also showed an increase in beneficial bacteria, including Bifidobacterium, Lactobacillus, Lactococcus, Bacillus subtilis, and butyrate-producing Eubacteria. Exactly what you would want to happen (kill the bad, boost the good).
PreforPro is a novel prebiotic that supports the growth of healthy bacteria in the gut through a mechanism that is not fiber or starch‐based.
Benefits include:
- it is efficacious in small doses (15mg) and starts working within hours (not days),
- it functions in both the small and large intestines,
- it does not cause flatulence or bloating,
- is not affected by varying gut environments,
- it works in conjunction with a broad spectrum of probiotic species (those normally found in the gut). We’ve included 5 probiotics that have been specifically tested to benefit from this phage complex; these probiotics are discussed below.
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
Lactobacillus plantarum
L. plantarum is a gram-positive oxygen tolerant anaerobic bacteria. It is commonly found in fermented vegetable foods (such as sauerkraut and kimchi) and plant matter.
- Non-Histamine Producing
- Non D-Lactate Producing
- May mitigate symptoms after antibiotic therapy
- Can produce the vitamin folate in the intestine
This strain has been shown in research studies to protect against intestinal permeability (leaky gut).
“These data demonstrate that pretreatment with L. plantarum 299v, which is a probiotic bacterium, protects against E. coli-induced increase in intestinal permeability, and that L. plantarum 299v alone has no influence on the intestinal permeability. Thus, this study supports the concept that probiotics may exert beneficial effects in the gastrointestinal tract.” — PMID #11911334
“administration of L. plantarum 299v counteracts the otherwise increased passage of mannitol across the small intestinal wall induced by a non-pathogenic strain of E. coli… L. plantarum 299v administered for one week in the drinking water could protect from E. coli-induced permeability… in a placebo controlled study of critically ill patients treated by antibiotics, L. plantarum 299v was shown to reduce the colonization of Clostridium difficile… L. plantarum 299v has been administered in randomized, double blinded, placebo controlled trials to IBS patients, where they experienced relief in their symptoms including significantly less bloating and abdominal pain” – PMID # PMC3257727
“L. plantarum in intestinal epithelial function and its therapeutic effects in the cellular and molecular mechanisms of intestinal barrier dysfunction and intestinal inflammation and justifies the use in inflammatory disorders, which is significant to both biotechnical and clinical fields. L. plantarum can protect against intestinal epithelial barrier dysfunction” – PMID # PMC2997994
An IBS & SIBO “… study has shown that 4 weeks of treatment with Lactobacillus plantarum improved symptoms such as pain and flatulence in IBS patients and these effects continued for 1 year. L. plantarum was found in the feces (84%) and rectal mucosa (34%) only in the treated group. This result showed that probiotics could alter the host gut microbiota composition with improvement of IBS symptoms… Another similar study in 40 patients with IBS significantly ameliorated abdominal pain and improved all IBS symptoms compared to placebo.” – PMID # PMC3155061
“L plantarum 299v, was shown to abolish increased intestinal permeability in rats exposed to Escherichia coli.” – DOI: 10.1016/j.jpeds.2004.06.068
“evidence from experimental animal studies consistently indicates that probiotics exert barrier-enhancing, antibacterial, immune-modulating, and anti-inflammatory effects, which all could be benefits in small intestinal bacterial overgrowth and intestinal failure… L plantarum 299v may either prevent or delay symptom recurrence after antibiotic therapy” — doi:10.1053/j.gastro.2005.11.046
“Lactobacillus plantarum constitutes an exception among lactobacilli, since it is capable of folate production” – PMC3257725
Lactobacillus rhamnosus
L. rhamnosus is a gram-positive beneficial bacteria strain commonly found in probiotics. It is also found in kefir and un-pasteurized milk. It is both acid and bile-stable and helps keep the environment of the gut healthy by producing l-lactic acid. Lactobacillus rhamnosus survives gastrointestinal transit and does not produce D-lactate (PMID # 16734098). Though not a true “resident” strain, it is thought to have the ability attach to the intestinal lumen and stay through several bowel movements. L. rhamnosus is one of the world’s most researched probiotic strains with over 800 scientific studies.
- Non-Histamine Producing
- Non D-Lactate Producing
- Mast Cell / Histamine Stabilizer
- Can help prevent Antibiotic-associated diarrhea
- Can produce Vitamin K2 in the intestine
“Median duration of diarrhoea was significantly shorter (P<0.001) in children who received L rhamnosus strain… One day after the first probiotic administration, the daily number of stools was significantly lower (P<0.001) in children who received L rhamnosus strain” – PMID # PMC1949444
We conclude that Lactobacillus (rhamnosus) GG supplementation is well tolerated and may reduce the frequency of severe diarrhoea and abdominal discomfort related to 5-FU-based chemotherapy. – PMID # PMC2360429
“Patients who received Lactobacillus had less grade 3 or 4 diarrhoea (22 vs 37%, P=0.027), reported less abdominal discomfort, needed less hospital care and had fewer chemotherapy dose reductions due to bowel toxicity. No Lactobacillus-related toxicity was detected… We conclude that Lactobacillus (rhamnosus) GG supplementation is well tolerated and may reduce the frequency of severe diarrhoea and abdominal discomfort related to 5-FU-based chemotherapy.” – PMID # 17895895
“Using meta-analyses, three types of probiotics (Saccharomyces boulardii, Lactobacillus rhamnosus GG, and probiotic mixtures) significantly reduced the development of antibiotic-associated diarrhea.” – PMID # 16635227
“L.rhamnosus JB-1 treatment lead to significant inhibition of mast cell mediator release in response to a range of stimuli including IgE mediated activation… These studies demonstrate that Ingestion of L.rhamnosus JB-1 leads to mast cell stabilization in rats and identify KCa3.1 as an immunomodulatory target for certain lactobacilli. Thus the systemic effects of certain candidate probiotics may include mast cell stabilization and such actions could contribute to the beneficial effect of these organisms in allergic and other inflammatory disorders.” – PMID # PMC3398942
Lactobacillus rhamnosus “have a potential protection effect against colon carcinogenesis; inducing apoptosis and ameliorating inflammation, and may hold a promise as bio-therapeutic dietary agent.” – PMID #27447122
“production of menaquinones, or vitamin K, which is a capacity that appears unique for L. rhamnosus among the Lactobacillus genus” – PMID #PMC4943194
Bifidobacterium bifidum
Produces no histamine and no d-lactic acid, Gram-Positive. Once babies are born, B. bifidum is one of the first colonizers of the human. In a healthy adult gut, this strain continues with us for life.
- Non-Histamine Producing
- Non D-Lactate Producing
- Protects the intestinal wall and reinforces the epithelial barrier function. Improves and thickens the mucus layer of the gut.
- Helps fight h.pylori and partially relieves damage it causes to the gastric tissue.
- Improves intestinal tight junctions, helps repair and prevent leaky gut.
“mucin breakdown activity as operated by B. bifidum could trigger the secretion of additional colonic mucin, thus increasing the thickness of the total amount of mucus layer covering the gut and so reinforcing the epithelial barrier function, which constitutes an important feature especially in those subjects affected by irritable bowel syndrome… The capacity to efficiently use mucus is a typical feature also of Akkermansia muciniphila, a human intestinal species that has been associated with healthy intestines and disease prevention” – PMID # PMC4140077
“These results, together with the diminished H. pylori pathogenicity ratio in animals treated with bifidobacteria, indicate that strain B. bifidum CECT 7366 is a potential probiotic, exerting in vivo antagonistic activity against H. pylori. Furthermore, in vivo assays have demonstrated that it partially relieves damage to gastric tissues caused by the pathogen.” – PMID # PMC3067243
“Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum… These results suggest that Bifidobacterium species enhance intestinal epithelial barrier function” – PMID # 25780093
“Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis (NEC)… administration of B. bifidum protects against NEC in the neonatal rat model. This protective effect is associated with reduction of inflammatory reaction in the ileum, regulation of main components of mucus layer, and improvement of intestinal integrity… B. bifidum treatment appears to enhance the development and formation of functional TJs… altered distribution pattern of these two TJ proteins contributes to increased intestinal permeability and disassembly of TJs leading to a breach in mucosal barrier in NEC animals. Treatment with B. bifidum prevents these pathological changes by protecting intestinal barrier integrity, thus blocking translocation of luminal toxins into systemic circulation.” – PMID # PMC2777452
“we showed that B. bifidum-induced restoration of epithelial TJ (Tight Junction) barrier may be attributed to increased production of acetate and formate, as demonstrated in cocultures of B. bifidum and Caco-2 cells… B. bifidum WU12 exerted the highest TER-restorative effect on Caco-2 cell monolayers among the tested Bifidobacterium strains.” – PMID # PMC4393161
Bifidobacterium longum
- Non-Histamine Producing
- Non D-Lactate Producing
- Possibly improves cognition
- Converts sugars to l-Lactic acid which has been shown to create an environment inhospitable to many bad bacterial strains and hospitable to beneficial strains.
- Promotes proper mast cell activation.
- Can help reduce inflammation in the gut.
- May benefit people with fructose malabsorption as B.longum utilizes excess fructose as a food source (doing so without harmful byproducts).
“Bifidobacterium longum alleviates food allergy through mast cell suppression. – 2016 PMID # 26433560 ”
“The effect of BL (Bifidobacterium longum) were evaluated first on two different models. Using ex vivo human skin explant model we found a statistically significant improvement versus placebo in various parameters associated with inflammation such as a decrease in vasodilation, oedema, mast cell degranulation and TNF-alpha release.” – PMID # 19624730
“Increasing evidence suggests that a brain-gut-microbiome axis exists, which has the potential to play a major role in modulating behaviour. However, the role of this axis in cognition remains relatively unexplored. Probiotics, which are commensal bacteria offering potential health benefit, have been shown to decrease anxiety, depression and visceral pain-related behaviours… B. longum 1714-treated mice made fewer errors than other groups, suggesting a better learning. In the fear conditioning, B. longum 1714-treated group also showed better learning and memory” – PMID # 25794930
“B. longum probiotics suppressed inflammatory destruction of the gut” – PMID # PMC3725482
“A number of studies have indicated that resistant carbohydrate is utilized by the bacteria in the colon in the biosynthesis of folate, particularly Bifidobacterium bifidum and Bifidobacterium longum… A number of other B-group vitamins that are synthesised by members of the microbiota and that may be produced in the gut include riboflavin, vitamin B12, niacin and pyridoxine” – PMID # PMC3571646
“inhibits the growth of C. albicans and some pathogenic bacteria.” – PMID # 18661679
“Short term synbiotic (Bifidobacterium longum) treatment of active UC resulted in improvement of the full clinical appearance of chronic inflammation in patients receiving this therapy.” – PMID # PMC1774839
“Gout is an acute inflammatory disease characterised by the presence of uric acid crystals in the joint… oral BL (Bifidobacterium longum) treatment reduced the inflammatory response in an experimental murine model of gout, suggesting it may be useful as an adjuvant treatment in patients with gout.” – PMID # 26322542
“The researchers found that mice colonized by one bifidobacterium subspecies, B. longum, were able to survive when fed the pathogenic bacteria E. coli O157, while GF mice without the bacteria died of infection within 7 days… The key actor in this mechanism is a carbohydrate transporter encoded by genes present in certain strains of bifidobacteria such as B. longum, which enables these bacteria to utilize fructose to produce acetate in the distal colon.” – DOI: 10.1038/nature09646
“Bifidobacterium longum may down-regulate IL-18 and IL-1β (proinflammatory cytokines) expressions… thus reduce the visceral hypersensitivity of PI-IBS (postinfectious irritable bowel syndrome).” – PMID # 26697916
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
Bacillus subtilis – DE111®
- Non-Histamine Producing
- Non D-Lactate Producing
- May improve villi health
- May mitigate symptoms after antibiotic therapy
- Spore forming, stays in the gut 30-60 days
- DE111 supports the normal breakdown of complex carbohydrates and fats, promoting proper digestion and nutrient absorption
- Communicates with intestinal cells to maintain gut barrier function [1]
- Crowds out bacterial pathogens and maintains healthy gut flora [2] [3]
- NIH Study Finds Probiotic Bacillus Eliminates Staphylococcus Bacteria [4]
Bacillus subtilis was discovered by German soldiers during WW2. A large number of German soldiers were dying of dysentery while fighting in African and little to no medicine was available. German scientists discovered that the locals had a treatment they had been using for at least hundreds of years, when someone got sick with dysentery they would eat a small amount of fresh camel dung. It was later discovered that this dung had a very large amount of a bacteria 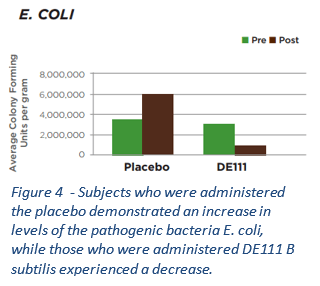 later named Bacillus subtilis. Bacillus subtilis was later cultured commercially and used by the German army to cure dysentery. After the war many western countries started using B. subtilis for gut and urinary tract diseases.
later named Bacillus subtilis. Bacillus subtilis was later cultured commercially and used by the German army to cure dysentery. After the war many western countries started using B. subtilis for gut and urinary tract diseases.
“B. subtilis promotes shallower crypt depth, which results in longer villi, greater villi surface area and more absorptive epithelial cells. Furthermore, shallower crypt depth promotes rapid epithelial turnover in response to inflammation from pathogenic bacteria… Changes in small intestinal morphology and in particular, increased villus height and VH: CD ratio in ducks fed diets supplemented with B. subtilis indicate improved gut health and digestive capacity” – PMID # PMC5143344
“Ingestion of significant quantities of B. subtilis is thought to restore the normal microbial flora following extensive antibiotic use or illness” – PMID # PMC99781
“The experiments showed that 70–90% of dietary-supplemented Bacillus spores germinate in the proximal part of the pig GI tract… Bacillus strains can temporarily remain in the GI system, but will be unable to permanently colonize the GI tract.” — doi:10.1111/j.1365-2672.2007.03633.x
“B. subtilis strain may be considered as non-pathogenic and safe for human consumption.” – PMID # 17934835
“CONCLUSIONS: Bacillus indicus and B. subtilis should be considered safe for oral use” – PMID # 18312567
“Bacillus subtilis R0179 used in several commercial probiotic products… Strains were subsequently screened for the presence of enterotoxins and virulence factors and were subjected to 28 days of repeated high-dose oral toxicity testing in rats. No risk factors or aberrant activities were identified using such a detailed approach. Thus, both microbes were deemed to pose low risk to the consumer and, therefore, safe for use as probiotics.” – PMID # 18449224
“Spores of Bacillus species are found in soil, dust, and water as well as in the air (27). Their primary reservoir though, has long been considered soil, and indeed, they can be found there in abundance… we have used a molecular approach to prove that orally administered B. subtilis spores germinate, proliferate, and then resporulate within the gut” — doi:10.1128/JB.188.7.2692–2700.2006
“The safety of Bacillus species has been extensively reviewed elsewhere (de Boer and Diderichsen, 1991; Ishibashi and Yamazaki, 2001; Logan, 2004; Osipova et al., 1998; Sanders et al., 2003; SCAN, 2000a) and most incidences of illness associated with Bacillus appear to result for opportunistic infections or miss-diagnosis. Extensive animal studies including acute and sub-chronic toxicity testing as well as in vitro studies have now been performed on a number of species, including B. subtilis var. Natto (Hong et al., 2008), Bacillus indicus (Hong et al., 2008), B. coagulans (Endres et al., 2009) and B. subtilis 2335 (Sorokulova et al., 2008) and B. licheniformis 2336 (Sorokulova et al., 2008). All appear to show no indicators of adverse effects. Studies are showing that these bacteria are able to grow within the intestinal tract and possibly be considered temporary residents. This is important because it shows that these bacteria are not foreigners but rather may exert a unique symbiotic relationship with their host.” – DOI: 10.1016/j.fm.2010.03.007
“A new study from National Institutes of Health scientists and their Thai colleagues shows that a “good” bacterium commonly found in probiotic digestive supplements helps eliminate Staphylococcus aureus, a type of bacteria that can cause serious antibiotic-resistant infections… the scientists colonized the gut of mice with S. aureus and fed them B. subtilis… given every two days eliminated S. aureus in the guts of the mice.” — NIH.GOV
Are you ready to order? If so, Click Here
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
- Thomas, Carissa M, and James Versalovic. “Probiotics-Host Communication: Modulation of Signaling Pathways in the Intestine.” Gut Microbes 1.3 (2010): 148–163. PMC. Web. 24 Feb. 2015. ↑
- Vacca A, Pantaleo G, Ronco M, Dammacco F. Chemoimmunotherapy for multiple myeloma using an intermittent combination drug schedule (melphalan + prednisone) and alternating course of B. subtilis spores. Chemioterapia. 1983;2:300–305 ↑
- Mazza P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm. 1994;133:3–18 ↑
* Though Phage Complete contains none of the allergy causing ingredients listed, it is manufactured in a plant that may process these ingredients. Though great care is taken to prevent cross-contamination this can’t be guaranteed.
[print-me]
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
All images posted by John Herron are either "Copyrighted John Herron", or are copyrighted by someone else and are used under license. So please don’t use them elsewhere, you’ll get in trouble.







 Phage Complete comes with a full 30 day money back guarantee, for U.S. purchases this includes the original shipping charges to you!
Phage Complete comes with a full 30 day money back guarantee, for U.S. purchases this includes the original shipping charges to you!